Contagious bovine pleuro-pneumonia (CBPP) or lung sickness in cattle, caused by Mycoplasma mycoides subsp. mycoides (Mmm) is truly an African disease, long eradicated from the developed world, which represents a considerable burden for cattle owners in many parts of Africa (EMPRES-AH, FAO, 2013), from Senegal and the Gambia in the west through Somalia in the east, and as far south as Namibia and Tanzania.
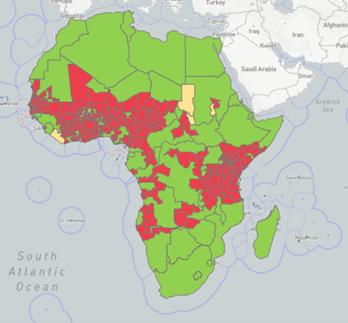
Map: WOAH-WAHIS composite map (2005 – 2019) of the distribution of CBPP in Africa (red = present; yellow = suspected). Map generated on 5 April 2022.
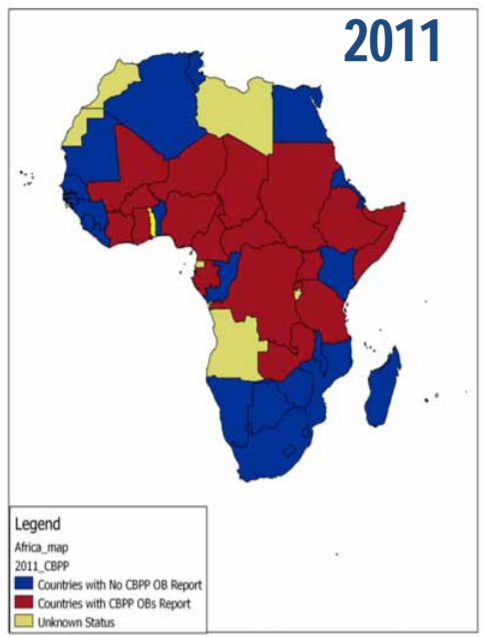
Map: Absence/presence of CBPP in 2011 (FAO/OIE/IAEA/IBAR, 2012)
In recent years, the disease has seen its area of spread increase in Africa (e.g. Senegal in West Africa, Gabon in Central Africa) and the number of outbreaks increase in areas where it was already present. It is currently being reported as present by around 18 countries (WAHIS, Jan – Jun 2019) with the latest outbreaks having been reported from Namibia (2021, 2020, 2019), Niger (2020) and the Gambia (2018).
As one of the listed diseases, subject to the procedure for official recognition of animal health status by the World Organisation for Animal Health (WOAH, founded as OIE), only four countries in Africa are currently officially free from CBPP, i.e. Botswana, Eswatini, South Africa (country-wide) and Namibia (zone located south of the Veterinary Cordon Fence, VCF). Namibia and Zambia are also the only countries having a WOAH endorsed official control programme for CBPP.
Several factors compound the control of CBPP: the fact that the disease is seen as a production disease, chronic and with rather limited mortality, that meat and meat products (excluding lungs) are regarded by WOAH as safe commodities according to the Terrestrial Animal Health Code (TAHC), irrespective of the disease status of the country or zone, that the disease is widely treated with antibiotics, mitigating the symptoms, but at the same time propagating the infection through carriers and -most importantly- the limited efficacy of the available vaccines, mainly based on the attenuated strains T1/44 and T1sr.
Though live attenuated vaccines (T1/44 and T1sr) are available, their protection is limited to maximum of 12 months, hence requiring considerable logistical efforts to attain protection at population level. An additional constraint to attain demonstrated absence of infection or disease is the need for animal identification and traceability systems to be in place.
As a result, CBPP can only realistically be controlled through a series of measures, one which is movement control, making it a truly transboundary animal disease. In a paper released in 1987, in the Rev. sci. tech. Off. int. Epiz., Provost et al. affirmed that the eradication of CBPP was possible on the condition that all cattle are vaccinated for several years in a row and that all clinically affected animals need to be emergency slaughtered. The latest guidance on CBPP dates back to 2003 (the FAO – OIE – AU/IBAR – IAEA Consultative Group on Contagious Bovine Pleuropneumonia, Third meeting “Towards Sustainable CBPP Control Programmes For Africa”, Rome, 12–14 November 2003 – http://www.fao.org/3/a-y5510e.pdf), demonstrating that CBPP control has become a neglected public good.
PDF - 180.50KB
PDF - 1.60MB
Picture (c) Vaccine and Infectious Disease Organization – International Vaccine Centre
WOAH Reference Laboratory
Dr Chandapiwa Marobela Raborokgwe
National Veterinary Laboratory
Private Bag 0035
Gaborone
BOTSWANA
Tel: +267 392 87 16
Email: [email protected]
FAO Reference Centre for technical assistance in quality control of veterinary vaccines
WOAH Collaborating Centre for the quality control of veterinary vaccines
Dr Nick Nwankpa
Pan African Veterinary Vaccines Centre (PANVAC)
African Union
P.o.box 1746, Debre Zeit,
ETHIOPIA
Tel: +251 – 11 4338001
Email: [email protected]
Email: [email protected]